A new class of pyrenyl solid-state emitters: 1-pyrenyl ynones. Synthesis via the Friedel–Crafts route, molecular and electronic structure and photophysical properties
Date
2014-06-15Author
Flamholc, Rafał
Plazuk, Damian
Zakrzewski, Janusz
Metivier, Remi
Nakatani, Keitaro
Makal, Anna
Wozniak, Krzysztof
Metadata
Show full item recordAbstract
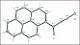
Friedel–Crafts acylation of pyrene with alkynoic acids in the presence of trifluoroacetic anhydride and triflic acid constitutes a direct and efficient route to 1-pyrenyl ynones. These compounds in chloroform solution emit fluorescence at longer wavelengths, with higher quantum yields and longer lifetimes than a typical saturated acylpyrene derivative, 1-acetylpyrene. Moreover, in contrast to 1-acetylpyrene, they are moderate solid-state emitters. Comparative DFT studies revealed strong stabilization of the LUMOs of 1-pyrenyl ynones in comparison to the LUMO of 1-acetylpyrene. The single-crystal X-ray structure of 1-(pyren-1-yl)but-2-yn-1-one showed π-interactions of pyrenyl moieties in the crystal lattice. Investigations of the solid-state fluorescence of this compound revealed emission from long-lived excited states, including excimer species.
Collections
The following license files are associated with this item:

